Behind KANJINTI®
IDENTICAL DOSING TO HERCEPTIN® IV
KANJINTI® PROVIDES IDENTICAL DOSING TO HERCEPTIN® IV ACROSS ALL INDICATIONS
Dosing similarity is one of several ways Amgen helps provide you and your patients with
a seamless
experience.1,2
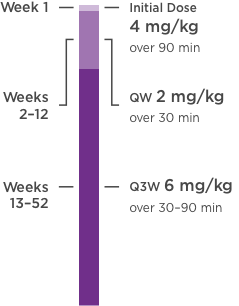
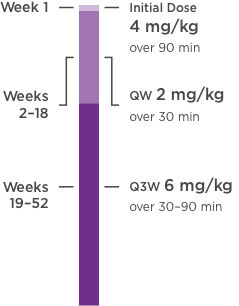
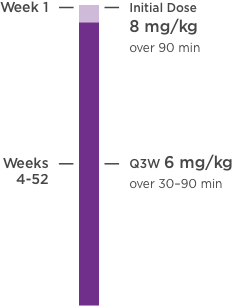
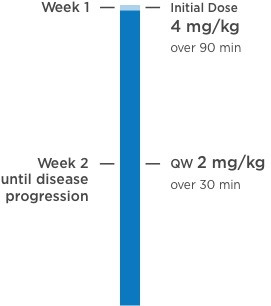
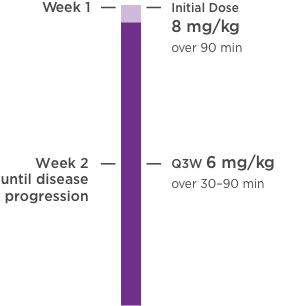
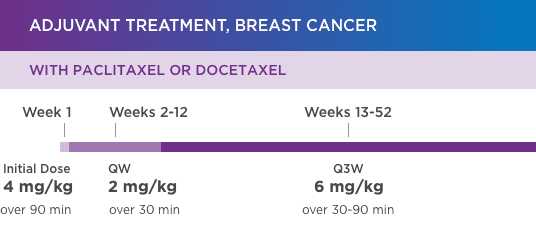
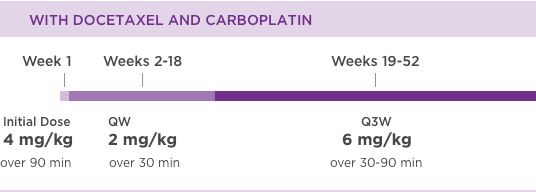
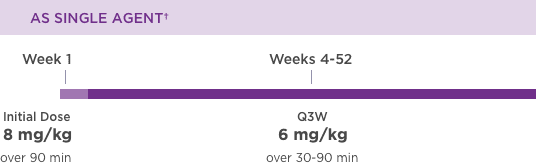
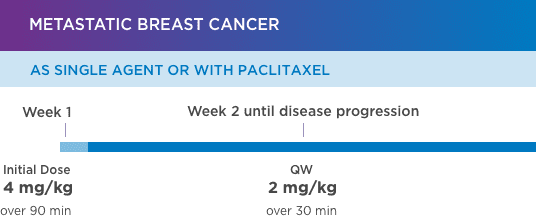
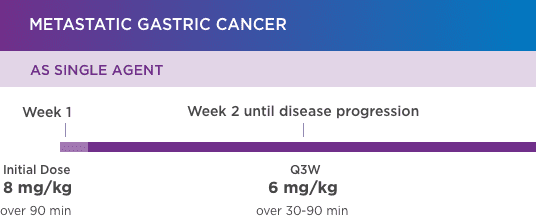
IV = intravenous; QW = once a week; Q3W = once every 3 weeks.
Please refer to accompanying full Prescribing Information for complete dosing and administration guidelines.




IV = intravenous; QW = once a week; Q3W = once every 3 weeks.
Please refer to accompanying full Prescribing Information for complete dosing and administration guidelines.
KANJINTI® has the same
dosing as Herceptin® IV
across all indications1,2
*Within 3 weeks following completion lof all chemotherapy.
†EU-sourced Herceptin® was used in the LILAC study. The KANJINTI® clinical pharmacokinetic (PK) study demonstrated equivalence between EU- and US-sourced Herceptin® and KANJINTI®.
*Within 3 weeks following completion of all chemotherapy.
†EU-sourced Herceptin® was used in
the LILAC study. The KANJINTI®
clinical pharmacokinetic (PK)
study demonstrated equivalence between
EU- and US-sourced Herceptin® and KANJINTI®.
